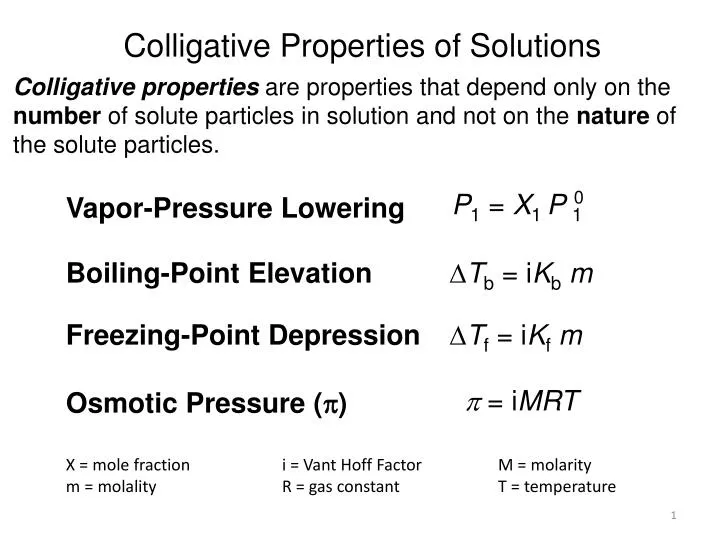
Dilute Solutions And Colligative Properties . Web describe colligative properties of solutions and correlate these with molar masses of the solutes; Web the properties of a solution are different from those of either the pure solute (s) or solvent. Web the colligative properties of a solution depend on only the total number of dissolved particles in solution, not on their chemical identity. Many solution properties are dependent upon. Web note that all four properties are defined by an equilibrium between the liquid solution and a solid, liquid, or gas phase of the pure solvent. Web by definition, one of the properties of a solution is a colligative property if it depends only on the ratio of the number of particles of solute and solvent in the.
from www.slideserve.com
Web the properties of a solution are different from those of either the pure solute (s) or solvent. Web note that all four properties are defined by an equilibrium between the liquid solution and a solid, liquid, or gas phase of the pure solvent. Web the colligative properties of a solution depend on only the total number of dissolved particles in solution, not on their chemical identity. Web by definition, one of the properties of a solution is a colligative property if it depends only on the ratio of the number of particles of solute and solvent in the. Many solution properties are dependent upon. Web describe colligative properties of solutions and correlate these with molar masses of the solutes;
PPT Colligative Properties of Solutions PowerPoint Presentation, free
Dilute Solutions And Colligative Properties Web describe colligative properties of solutions and correlate these with molar masses of the solutes; Web note that all four properties are defined by an equilibrium between the liquid solution and a solid, liquid, or gas phase of the pure solvent. Web describe colligative properties of solutions and correlate these with molar masses of the solutes; Many solution properties are dependent upon. Web by definition, one of the properties of a solution is a colligative property if it depends only on the ratio of the number of particles of solute and solvent in the. Web the properties of a solution are different from those of either the pure solute (s) or solvent. Web the colligative properties of a solution depend on only the total number of dissolved particles in solution, not on their chemical identity.
From www.scribd.com
Chapter 13 Dilute Solution and Colligative Properties PDF Dilute Solutions And Colligative Properties Many solution properties are dependent upon. Web the properties of a solution are different from those of either the pure solute (s) or solvent. Web describe colligative properties of solutions and correlate these with molar masses of the solutes; Web by definition, one of the properties of a solution is a colligative property if it depends only on the ratio. Dilute Solutions And Colligative Properties.
From askfilo.com
7. The colligative properties of a dilute solution depend upon (a) the n.. Dilute Solutions And Colligative Properties Web the colligative properties of a solution depend on only the total number of dissolved particles in solution, not on their chemical identity. Web by definition, one of the properties of a solution is a colligative property if it depends only on the ratio of the number of particles of solute and solvent in the. Web describe colligative properties of. Dilute Solutions And Colligative Properties.
From www.youtube.com
Dilute Solutions & Colligative Properties Pt 2 YouTube Dilute Solutions And Colligative Properties Web the colligative properties of a solution depend on only the total number of dissolved particles in solution, not on their chemical identity. Web the properties of a solution are different from those of either the pure solute (s) or solvent. Web by definition, one of the properties of a solution is a colligative property if it depends only on. Dilute Solutions And Colligative Properties.
From www.askiitians.com
Colligative Properties Of Dilute Solutions Study Material for IIT JEE Dilute Solutions And Colligative Properties Web the colligative properties of a solution depend on only the total number of dissolved particles in solution, not on their chemical identity. Web describe colligative properties of solutions and correlate these with molar masses of the solutes; Many solution properties are dependent upon. Web the properties of a solution are different from those of either the pure solute (s). Dilute Solutions And Colligative Properties.
From www.scribd.com
C 2Y Dilute Solution and Colligative Properties Assignment 1 Dilute Solutions And Colligative Properties Web describe colligative properties of solutions and correlate these with molar masses of the solutes; Web the colligative properties of a solution depend on only the total number of dissolved particles in solution, not on their chemical identity. Many solution properties are dependent upon. Web the properties of a solution are different from those of either the pure solute (s). Dilute Solutions And Colligative Properties.
From www.slideserve.com
PPT Colligative Properties PowerPoint Presentation, free download Dilute Solutions And Colligative Properties Web the colligative properties of a solution depend on only the total number of dissolved particles in solution, not on their chemical identity. Web note that all four properties are defined by an equilibrium between the liquid solution and a solid, liquid, or gas phase of the pure solvent. Web the properties of a solution are different from those of. Dilute Solutions And Colligative Properties.
From www.studypool.com
SOLUTION Dilute solution colligative properties physical chemistry Dilute Solutions And Colligative Properties Web describe colligative properties of solutions and correlate these with molar masses of the solutes; Web the colligative properties of a solution depend on only the total number of dissolved particles in solution, not on their chemical identity. Web note that all four properties are defined by an equilibrium between the liquid solution and a solid, liquid, or gas phase. Dilute Solutions And Colligative Properties.
From www.studypool.com
SOLUTION Dilute solution colligative properties physical chemistry Dilute Solutions And Colligative Properties Web the colligative properties of a solution depend on only the total number of dissolved particles in solution, not on their chemical identity. Web the properties of a solution are different from those of either the pure solute (s) or solvent. Many solution properties are dependent upon. Web describe colligative properties of solutions and correlate these with molar masses of. Dilute Solutions And Colligative Properties.
From www.youtube.com
Dilute Solutions & Colligative Properties Pt 5 YouTube Dilute Solutions And Colligative Properties Web note that all four properties are defined by an equilibrium between the liquid solution and a solid, liquid, or gas phase of the pure solvent. Many solution properties are dependent upon. Web the colligative properties of a solution depend on only the total number of dissolved particles in solution, not on their chemical identity. Web by definition, one of. Dilute Solutions And Colligative Properties.
From sciencenotes.org
What Are Colligative Properties? Definition and Examples Dilute Solutions And Colligative Properties Web the colligative properties of a solution depend on only the total number of dissolved particles in solution, not on their chemical identity. Many solution properties are dependent upon. Web note that all four properties are defined by an equilibrium between the liquid solution and a solid, liquid, or gas phase of the pure solvent. Web describe colligative properties of. Dilute Solutions And Colligative Properties.
From dokumen.tips
(PDF) DILUTE SOLUTION & COLLIGATIVE PROPERTIESASSIGNMENT.doc DOKUMEN Dilute Solutions And Colligative Properties Web describe colligative properties of solutions and correlate these with molar masses of the solutes; Web the properties of a solution are different from those of either the pure solute (s) or solvent. Many solution properties are dependent upon. Web note that all four properties are defined by an equilibrium between the liquid solution and a solid, liquid, or gas. Dilute Solutions And Colligative Properties.
From chem.libretexts.org
12.4 Colligative Properties of a Dilute Solution Chemistry LibreTexts Dilute Solutions And Colligative Properties Many solution properties are dependent upon. Web note that all four properties are defined by an equilibrium between the liquid solution and a solid, liquid, or gas phase of the pure solvent. Web the colligative properties of a solution depend on only the total number of dissolved particles in solution, not on their chemical identity. Web the properties of a. Dilute Solutions And Colligative Properties.
From www.studypool.com
SOLUTION Handwritten notes for dilute solution and colligative Dilute Solutions And Colligative Properties Web by definition, one of the properties of a solution is a colligative property if it depends only on the ratio of the number of particles of solute and solvent in the. Web the properties of a solution are different from those of either the pure solute (s) or solvent. Web the colligative properties of a solution depend on only. Dilute Solutions And Colligative Properties.
From www.studocu.com
Solution and Colligative Properties UNIT 8 DILUTE SOLUTION AND Dilute Solutions And Colligative Properties Web the colligative properties of a solution depend on only the total number of dissolved particles in solution, not on their chemical identity. Web describe colligative properties of solutions and correlate these with molar masses of the solutes; Web the properties of a solution are different from those of either the pure solute (s) or solvent. Web by definition, one. Dilute Solutions And Colligative Properties.
From www.askiitians.com
Colligative Properties Of Dilute Solutions Study Material for IIT JEE Dilute Solutions And Colligative Properties Web describe colligative properties of solutions and correlate these with molar masses of the solutes; Web by definition, one of the properties of a solution is a colligative property if it depends only on the ratio of the number of particles of solute and solvent in the. Many solution properties are dependent upon. Web the properties of a solution are. Dilute Solutions And Colligative Properties.
From www.youtube.com
Dilute Solution Colligative Property Lecture 6 Osmotic Pressure Dilute Solutions And Colligative Properties Web the colligative properties of a solution depend on only the total number of dissolved particles in solution, not on their chemical identity. Web note that all four properties are defined by an equilibrium between the liquid solution and a solid, liquid, or gas phase of the pure solvent. Many solution properties are dependent upon. Web the properties of a. Dilute Solutions And Colligative Properties.
From slideplayer.com
Colligative Properties ppt download Dilute Solutions And Colligative Properties Web the properties of a solution are different from those of either the pure solute (s) or solvent. Many solution properties are dependent upon. Web note that all four properties are defined by an equilibrium between the liquid solution and a solid, liquid, or gas phase of the pure solvent. Web the colligative properties of a solution depend on only. Dilute Solutions And Colligative Properties.
From www.youtube.com
COLLIGATIVE PROPERTIES OF DILUTE SOLUTIONS YouTube Dilute Solutions And Colligative Properties Web note that all four properties are defined by an equilibrium between the liquid solution and a solid, liquid, or gas phase of the pure solvent. Web by definition, one of the properties of a solution is a colligative property if it depends only on the ratio of the number of particles of solute and solvent in the. Web describe. Dilute Solutions And Colligative Properties.